- HOME
- WHO WE ARE
- NEWS AND DATES
- GOODEIDS
- PHYLOGENY
- ARTIFICIAL KEY
- GOODEID SPECIES
- BIOLOGY
- ENVIRONMENT
- CONSERVATION
- PROFUNDULIDS
- MEMBERS AREA
Crenichthys baileyi
English Name:
White River Springfish
Original Description:
GILBERT, C. H. (1893): Report on the fishes of the Death Valley expedition collected in southern California and Nevada in 1891, with descriptions of new species. North American Fauna Nr. 7, part II, Washington: pp 229 - 384
Holotype:
Collection-numbers: United States National Museum, Cat. No. USNM-46110 (6 specimens), Museum of Comparative Zoology (Harvard University), Cat No. MCZ-5996 (2 specimens), Syracuse University, Cat. No. SU-709 (3 specimens).
The types are 11 immature specimens (< 20mm), collected by Clinton H. Merriam and Vernon O. Bailey on May 25th, 1891.
As Gilbert's types of this species were immature, Williams and Wilde (1981) decided to chose two Topotypes from Ash Spring, one of each sex, to serve as representatives of the species. As male Topotype serves a specimen of 30.1mm standard length, as female one a specimen of 35,4mm SL, both deposited in the Museum of Zoology in Michigan under the Cat. No. UMMZ-203331 and collected with 29 additional specimens (Arizona State University, ASU-5196) of 21 to 44mm SL by the later president of the Desert Fishes Council, James E. Deacon on June 10th, 1967.
The left picture shows the Topotypes from Williams and Wilde, the right one the Hiko Spring in 1861. This spring locates about 15km north of Ash Spring and is the type locality of a population of Crenichthys baileyi that Williams and Wilde described exactly 120 years after this picture was taken as C. baileyi grandis:
Terra typica:
Gilbert didn't name a precise type locality, the only information available according the types is "Pahranagat Valley, Nevada".
According to the terra typica, here the appraisal of Willams and Wilde (1981): "The failure of Gilbert to list the specific locality of collection in Pahranagat Valley presents a problem since three springs in the valley, Ash, Crystal, and Hiko, harbored populations of springfish. Hiko would seem to be eliminated as the type locality since it is somewhat removed from the river bed and therefore the route of travel. The area near Ash Spring was frequented by travelers at that time and probably was a stopping place for Merriam and Bailey. That Merriam and Bailey collected fishes at Ash Spring is supported by the report of their collection of Rhinichthys in a spring of 36.11°C as described by Gilbert (1893). Ash spring is the only spring of that temperature in Pahranagat Valley, and therefore, the probable type locality of Crenichthys baileyi."
Etymology:
The species' name honours one of the collectors of the type material and member of the Death Valley Expedition in 1891, Vernon Orlando Bailey.
The genus was erected by Hubbs in 1932 with the generic name refering to the typical habitat of this genus, namely springs. It is derived from the ancient Greek with κρήνη (kréne or créne) meaning spring and ἰχθύς (íchthús, íchthýs), the Greek word for fish. The name of the genus simply means "Springfish".
Synonyms:
Cyprinodon macularius baileyi Gilbert, 1893
Cyprinodon baileyi Jordan & Evermann, 1896
Distribution and ESU's:
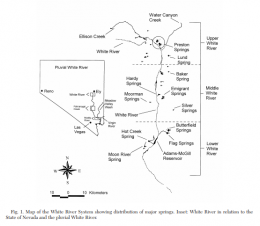
Crenichthys baileyi historically occured in a handfull of springs in the headwaters of the White River in Preston, White Pine County, then in few hot water springs in right affluents of the White River further downstream in Nye County, and three more warm spring areas along the Pahranagat Wash above Alamo, Lincoln County. Fish in and around five warm water springs in the headwaters of the Muddy River, Clark County probably belong to a separate species (Crenichthys cf. baileyi).
ESU ist short for Evolutionarily Significant Unit. Each unit expresses an isolated population with different genetic characteristics within one species. ESU's can be defined by Molecular genetics, Morphology and/or Zoogeography and help in indicating different phylogenetic lineages within a species. The abbreviation for an ESU is composed of three letters of the genus, followed by the first two letters of the species name and an ongoing number in each species.
In the subfamily Empetrichyinae, no ESU's were in use so far. To align with the livebearing subfamily Goodeinae, the GWG wants to bring a system in use based on John Lyons' naming for this subfamily. In Crenichthys nevadae, we actually (until the new species is described) distinguish five ESU's based on genetics and biogeography. Creba1 encompasses the type population from Ash Spring (corresponding with C. b. baileyi), Creba2 the neighbouring one from Crystal and Hiko Springs (C. b. grandis). We suggest Creba3 for the fish from Preston (C. b. albivallis) and Creba4 for the fish from Moon, Moorman and Hot Springs (C. b. thermophilus). Those last two ESU's are genetically almost identical, nevertheless we place them in two separate ESU's for not violating the existing system with subspecies too much, but also for taking account of the geographic separation. The fifth and last ESU, Creba5, we want to bring in use for the Muddy River populations (C. b. moapae), that represents a subspecies that probably deserves full species rank (for more details about these fish, go to Crenichthys cf. baileyi).
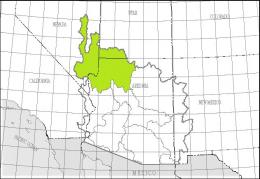
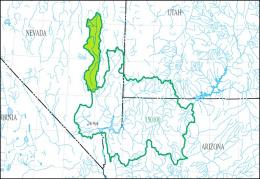
The left map shows the Lower Colorado-Lake Mead Accounting Unit (HUC 150100) of the Lower Colorado Basin Region (HUC 15), the right map the White River Cataloging Unit (HUC 15010011):
Status :
International Union for Conservation of Nature (IUCN): Endangered
U.S. Fish & Wildlife Service (ECOS): listed per subspecies
C.b.albivallis: not listed
C.b.baileyi: Endangered
C.b.grandis: Endangered
C.b.thermophilus: not listed
Habitat:
Crenichthys baileyi inhabits different springs that vary considerably in temperature and minimum dissolved oxygen values with these values being relatively constant within each spring. So after Hubbs and Hettler (1964) in Williams and Wilde (1981), Preston Big Spring was quite cool with a temperature of 21°C while Hiko and Crystal Springs were a bit warmer (26 to 27°C). Ash Springs (almost 36°C in the headpool) and the warm springs along the White River (31.3 to 37°C) were warmest. The White River Springfish is located in two main distribution centers. One is the Pahranagat Valley with three distinct spring systems being populated with C. b. baileyi and C. b. grandis sensu Williams and Wilde, the other one the White River Valley with a handful of springs in Preston (C. b. albivallis sensu Williams and Wilde), and three distinct spring areas along the White River itself (C. b. thermophilus sensu Williams and Wilde). We take the description of the habitats of the Pahranagat Valley from the Recovery Plan for the Aquatic and Riparian Species of Pahranagat Valley (U.S. Fish & Wildlife Service, 1998), and concerning the White River habitats, those of C. b. albivallis sensu Williams and Wilde from the Status Report from the Preston White River Springfish (Scoppettone & Rissler, 2002), both slightly modified.
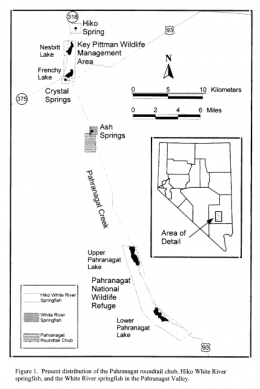
Ash Spring sis the southernmost, largest, and warmest of the three spring systems. Located 14.5km N of Alamo, Nevada, Ash Springs may have been the least altered historically, because it was the home of the Ash Ute Indians who were hunters rather than cultivators (Courtenay et al., 1985). It later became an important source of water for travelers. Ash Springs consists of at least seven springs which issue from a contact between alluvium and bedrock (Garside & Shilling, 1979). The springs have a common outflow stream, which has been impounded by construction of U. S. HW 93, and now forms a large pool. The spring pool provides good stream flow when the gate controlling the water level is open. Ash Springs was historically a stream with continuous flow before it was modified into the existing deep convoluted pool. Below the highway, the outflow stream flows southwest to join the outflow stream from Crystal Spring. From this point on, the stream is referred to as the Pahranagat Ditch. Ash Springs water temperatures range from 31 to 36°C, and mean discharge is 0.56m³/sec (Tuttle et al., 1990). The overall water quality for this site is excellent with only one recorded incident of elevated potassium level in 1934. Dissolved oxygen concentrations fluctuate between 1.8 and 5.1mg/l seasonally (Tuttle et al., 1990). The pH level has changed from slightly acidic (6.4) in 1935 to slightly basic (8.1), where it has remained for the past 3 decades. Ash Springs now form a large, convoluted pool, 0.4km long and 0.5 -2.0m deep (Tuttle et al., 1990). The bottom consists of sand and silt with locally dense submergent vegetation and algal mats. A thick canopy of willow (Salix sp.) and ash trees (Fraxinus sp.) border the eastern bank while the westside is more sparsely vegetated with willow, ash, and grasses.
Hiko Spring is the northernmost and the third largest spring in Pahranagat Valley (Garside & Schilling, 1979). The outflow stream from Hiko Spring was probably first redirected and impounded in 1865 to provide water for the silver stamp mills in the area, and secondarily created Nesbitt and Frenchy Lakes (Courtenay et al., 1985). Previously referred to as Hatch and Shutt’s Lakes, these lakes are now part of Nevada Division of Wildlife’s Key Pittman Wildlife Management Area. Today, the water from Hiko Spring is used for agricultural and municipal purposes. Previously diverted into concrete ditches, the entire outflow stream is now captured in underground pipes, which transport the water to nearby agricultural lands. The only surface water remaining is an impoundment at the spring source and a small marsh created by seepage from the spring pool. Hiko Spring maintains a temperature of 27°C, although a maximum temperature of 32°C was recorded in 1934. The water issues from a contact between alluvium and dolomite (Garside & Shilling, 1979), with a mean flow of 0.167m³/sec. The water issuing from the spring source is generally of good quality, although safe drinking water standards were exceeded during the 1930s and 1940s. In 1934, potassium concentrations were three times the safe drinking level, and in 1943 and 1944 boron levels rose above levels considered harmful to humans (American Public Health Association et al., 1985). Additionally, a high concentration of sodium in the spring water during 1940-42 may have harmed soil permeability (American Public Health Association et al., 1985). The water is slightly basic with a pH of 8.
Crystal Spring, located 27km NW of Alamo, Lincoln County, Nevada, is the second largest of the spring systems in the valley. Crystal Spring has been the most intensely disturbed by habitat alterations. It consists of at least two individual springs; one flows from an orifice in limestone bedrock and the other from a contact between alluvium and bedrock (Garside & Shilling, 1979). The Pahranagat Indians modified this spring for agricultural use before the first European settlement was established. The alteration was a ditch 2.4m wide, 1.8m deep, extending for several kilometers. The spring has since been altered continually foragricultural uses (Courtenay et al., 1985). In the 1880s all the spring outflows were further modified to provide additional water for agricultural use. The water level in Crystal Spring is controlled by a gate that directs flow into either ofthe two outflows. The main outflow (the historical headwaters of the Pahranagat Creek) continues for approximately 900m before flowing into a concrete irrigation channel, with five diversion boxes and seven outlet concrete channels (four to the east and three to the west). The riparian corridor along the main concrete channel is minimal. Farther downstream, the water flows back into an earthen channel. Portions ofthis channel have previously been trenched, but most areas appear to have been undisturbed for several years. Flow in this channel is periodically interrupted by agricultural diversions, however these diversions do not commonly cause desiccation ofthe entire flow. The last portion ofthe Crystal Spring outflow is an earthen irrigation ditch extending 5.8km and averaging 1m in width. This portion connects to the Ash Springs outflow; however, for much of the year only the upper 4.8km of the ditch contains water. The smaller outflow, created to provide water for nearby agriculture, conveys water intermittently, and thus offers little habitat for the Springfish. Crystal Spring impoundment allows for diversion of the entire spring flow into either the natural channel or the earthen irrigation ditch. The water level in the spring pool is lowered significantly when the natural channel is used, and it fluctuates throughout the irrigation season. Crystal Spring discharge varied between 0.169m³/sec in 1912 to 0.31m³/sec in 1989. Water temperature, which was warmer for the earlier part of this century, has cooled by several degrees in recent years. Overall water quality is good with a few exceptions. As in Hiko Spring, the potassium concentration exceeded safe drinking water standards in 1934. In 1936, chloride concentration was 69mg/l, which may be damaging to plant growth.The pH of Crystal Spring varies between neutral (7.0) to slightly basic (8.2). The dissolved oxygen levels in Crystal Spring ranges from 1.3 to 6.4mg/l depending on the season (Tuttle et al., 1990). The main channel ofthe outflow has a much greater dissolved oxygen concentration (6.5 to 15.7mg/l) than the created irrigation ditch (3.6 to 5.9mg/l, Tuttle et al., 1990). Dense vegetation, consisting mostly of the nonnative aquatic weed watercress (Nasturtium officinale), lines the sand and silt bottom of the spring pools. The main outflow has a maximum depth of 1.5m, width between 10 and 30m, and extends approximately 900m before discharging into a concrete irrigation ditch (Tuttle et al., 1990). This reach is also characterized by dense aquatic vegetation and silty substrate. The southern ditch off ofthe spring pool is much shallower and narrower, has very little vegetation, and has a silt substrate.
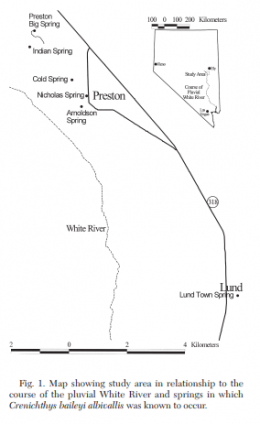
Springs with C. b. albivallis (Preston Big, Indian, Arnoldson, and Nicholas) are within a 2km radius and have a constant water temperature of about 21°C. Of those, Preston Big Spring is the northernmost and has the greatest discharge, 1,090m³/min (Garside and Schilling 1979). The pH ranged from 7.5 to 7.8 during the sampling period and the water was hard, 184 to 208 mg/1 with moderate amounts of oxygen, 2.7 to 5.0 mg/l. It flows south 524m in an earthen ditch before entering a pipe. The upstream reach is slow and wide, the downstream shallow and fast. The banks are lined with big sage (Artemesia tridentata). Indian Spring, about 0.6km southwest of Preston Big Spring, issues at 0.12m³/min. It flows more than 500m southeast in an excavated channel before discharging into a shallow reservoir. The channel is choked with bulrush (Scirpus sp.) along most of its course. The outflow of Arnoldson Spring travels 128m southeast before entering a pipe. Its discharge is about 0.24m³/min, and several cottonwoods (Populus sp.) border the channel. Nicholas Spring has the least amount of habitat available to C. b. albivallis; it flows only 10m before entering a pipe. Its flow is frequently manipulated, and its character fluctuates from pool to riffle. A large willow tree (Salix sp.) shades much of this reach, and discharge from the spring source is about 0.18m³/min.
Crenichthys baileyi thermophilus inhabits clear springs with water temperatures of approximately 33–37°C in the southern end of the White River. Hot Creek Spring is about 10m in diameter, artificially framed by big rocks and boulders, whereas the outflow is outlined by short grassy riparian vegetation.
Biology:
Adult White River Springfish are found at varying depths, from 0.4 to 1.7m, but prefer deeper water (1.1m). Juvenile White River Springfish will also use all depths, but generally occur in shallower (0.64m) water and are more vertically dispersed. Larval White River Springfish restrict their movement to the top of the water column (0 to 0.6m deep) and are found most frequently at 0.35m. All age classes of White River Springfish are present in areas of calm water (Tuttle et al., 1990). White River Springfish and Hiko White River Springfish are uniquely adapted for surviving in environments of extreme temperatures and low dissolved oxygen content (Hubbs & Hettler, 1964). The ability of Springfish to adaptively thermoregulate by moving in and out of areas of extreme temperatures, which would be lethal under extended exposure, and to live in water with a broad range of temperatures, has enabled them to survive in areas deemed too hostile for other fish species (Hubbs & Hettler, 1964). Deacon and Minckley (1974) defined Springfish spawning as asynchronous, i.e. individual females will spawn at different times of the year. Most females average two spawning periods a year, while the spawning season of the entire population extends over a long period of time each year. The period of spawning activity may be regulated by the primary productivity (production of food) in the spring system (Schoenherr, 1981).
Diet:
Crenichthys baileyi is an opportunistic omnivore. Studies concerning the gut contents of C. b. thermophilus showed a high percentage of invertebrates, more than 75% in summer and more than 40% in winter. C. b. albivallis from Preston Big Spring showed a preference for filamentous algae, diatoms and vascular plants, with digestive tract contents averaging 52.2% plant material, 15.3% animal, and 32.2% detritus. However, this subspecies was an opportunistic feeder, with some individuals consuming large quantities of Chironomidae and Trichoptera larvae. The mean ratio of digestive tract length to standard length was 1.43 in this subspecies also indicating omnivorous feeding habits.
Size:
The maximum known SL is 65mm (Willims & Wilde, 1981).
Colouration:
Young Crenichthys baileyi look very similar despite of the locality, while adults may differ significantly. Young and females show typically two black lines or rows of dots forming lines, one lateral and a second, shorter one, abdominal. In grown up males, this lines are typically overlaid by colouration. So male fish from Ash Springs are laterally orange or golden while the back is light or dark grey, unpaired fins dusky with broad black terminal bands. Fish from Hiko and Crystal Springs are lighter in colour, more yellow or yellowish with the caudal fin being brightly orange in big males. Males from the hot springs don't differ much from females, they stay more or less creme or white, the back a bit darker and greenish, but courthing males can change the colour to almost black. Preston White River Springfish are similar coloured to Hiko White River Springfish, also yellow or golden and displaying yellow to orange unpaired fins. In some characters, the colouration is similar over all populations. So is the back always darker, mostly coloured olivaceous or grey, and the sides have this dark lateral line that reaches from the eye to the caudal fin. The belly shows a greyish yellow with a dark abdominal line. The back is marked with a dark medial line reaching from the head to the dorsal fin. During courthisp, the colours become more intense and the black medial line contrats with a striking coloured back. The intensity of the colouration may be realized in varying degrees and depends on subspecies, sex, age and mood.
Sexual Dimorphism:
The sexes differ mainly in shape, fins and colouration. Males are higher in body and appear stronger, anal and dorsal fins are longer and have a more sailfin shape while the these fins of females are shorter and the fins are rather shaped like a rhombus. Male unpair fins express a broad dark to black terminal band and are coloured more intensively while those of females stay mainly clear or are slightly coloured. Males have their lateral band broader than females but less distinct and the medial stripe in males is darker and more prominant, especially during courtship.
Remarks:
Jack E. Williams and Gene R. Wilde published in 1981 a paper about the taxonomy and morphology of isolated populations of the White River Springfish. As part of this study, the authors decided to describe five subspecies that today would rather qualify for different lineages or ESU's instead of subspecies. However, as the subspecies system is still in use, we bring here more information about the Holotypes, the type localities and about the Etymology of species epithets of three of them: Crenichthys baileyi albivallis, grandis and thermophilus. The fourth one, Crenichthys baileyi baileyi is already representing the species in the chapters above while the fifth subspecies, Crenichthys baileyi moapae, will probably be described as a separate species. Information about this fish, you will be able to find here: Crenichthys cf. baileyi. All subspecies were described in:
Crenichthys baileyi albivallis
The Collection number of the Holotype of this subspecies is: Unversity of Michigan Museum and Zoology, Cat. No. UMMZ-203332. It is an adult male of 42.mm standard length, collected together with a female Allotype (Cat. No. UMMZ-203333) of 36mm SL and 28 Paratopotypes (Univeristy of Nevada, Las Vegas, Cat. No. UNLV-F-952), 30 to 48.7mm long. They were sampled in Preston Big Spring, White Pine County, Nevada, by James E. Deacon on October the 10th, 1966. The subspecies epithet can be derived from the Latin with albus meaning white and vallis meaning valley. Therefore its name can be translated with "white valley" and is a reference to the river basin the type locality belongs to, the White River.
Crenichthys baileyi grandis
The Collection number of the Holotype of this subspecies is: Unversity of Michigan Museum and Zoology, Cat. No. UMMZ-203336. It is a mature male of 45.5mm standard length, collected together with a female Allotype (Cat. No. UMMZ-203337) of 45.4mm SL and 29 Paratopotypes (Cat. No. ASU-3942), 31.3 to 65.5mm long. They were sampled in Hiko Spring, Lincoln County, Nevada, by James E. Deacon on the 3rd and 4th of June, 1966. Additional Paratopotypes are 44 specimens (Cat. No. UNLV-142) of 26.4 to 40.3 mm SL, collected on June the 4th, 1964, by J. E. Deacon, and 40 specimens (Cat. No. UMMZ-124998), part of a collection of 1,626 specimens of 13 to 71 mm SL, collected on August 28th, 1938, by Carl Leavitt Hubbs. The subspecies epithet can be derived from the Latin with the adjective grandis meaning big. The authors refered with this name to the size of this subspecies, that appeared signifícantly bigger in their studies than the other ones, naming it simply "the big one".
Crenichthys baileyi thermophilus
The Collection number of the Holotype of this subspecies is: Unversity of Michigan Museum and Zoology, Cat. No. UMMZ-203334. It is an adult male of 32.4mm standard length, collected together with six Paratopotypes (Cat. No. UNLV-124), 15.8 to 29.8mm SL by James E. Deacon on April 4th, 1965 in Mormon Spring, Nye County, Nevada. A female Allotype (Cat. No. UMMZ-203335) of 31.6mm SL was collected by the same person together with 12 Paratopotypes (Cat. No. UNLV-123) of 191 to 33.6mm SL on January 29th in the same year. Additional Paratopotypes collected from Mormon Spring are as follows: four specimens (Cat. No. UNLV-121) of 16.8 to 32.1 mm SL and six specimens (Cat. No. UNLV-122) of 18.5 to 30mm SL. The subspecies epithet can be derived from the Ancient Greek with Θερμός (thermós) meaning hot or warm and φίλος (phílos) meaning dear or beloved. The subspecies' name could therefore be translated with "loving it hot", a clear reference to the hot springs where they found this subspecies.
The left picture shows the types of Crenichthys baileyi albivallis, the one in the middle those of C. b. grandis, and the right one those of C. b. thermophilus:
The following texts about the situation of Springfish in Ash, Hiko and Crystal Spring were mainly taken from the Recovery Plan for the Aquatic and Riparian Species of Pahranagat Valley (U.S. Fish & Wildlife Service, 1998, slightly modified): White River Springfish are found throughout the Ash Springs pool with infrequent occurrences in the outflow stream (Tuttle et al., 1990). Historically, White River Springfish inhabited Ash Springs and its outflow stream and were considered common in these areas. With the introductions of mosquitofish (Gambusia affinis) in 1963, and convict cichlid (Amatitlania nigrofasciata), shortfin molly (Poecilia mexicana) and sailfin molly (Poecilia latipinna) in 1964, White River Springfish experienced a population decline. Additionally, Ash Springs is a very popular recreation area, primarily for swimming purposes. From 1986 through 1989, the pool was drained annually and algal growth was removed, keeping White River Springfish numbers low. In recent years, the habitat manipulations stopped though the swimming continued primarily in the northern and southern ends of the spring pool, allowing White River Springfish to establish a stable to increasing population. Swimming does not appear to impact the White River Springfish as much as previously thought as long as areas with little or no swimming are provided. Nonnatives continue to impact the White River Springfish population in the spring. Hiko White River Springfish were common in Hiko Spring and its outflow stream until 1963, when the outflow stream was modified for irrigation and mosquitofish were introduced (Courtenay et al., 1985). Shortfin mollies and largemouth bass (Micropterus salmoides) were first observed in Hiko Spring in February 1965, after which the Hiko White River Springfish population again declined (Courtenay et al., 1985). By 1967 Hiko White River Springfish had been extirpated from Hiko Spring and its outflow stream (Minckley & Deacon, 1968, Deacon, 1979, Williams & Wilde, 1981, Courtenay et al., 1985). Hiko White River Springfish collected from Crystal Spring were spawned at the University of Nevada, Las Vegas, and the resultant progeny were released into Hiko Spring in 1984. Convict cichlids had also been introduced into Hiko Spring by 1984 (Courtenay et al., 1985). Despite the presence of nonnative fishes, Hiko White River Springfish reestablished a population at Hiko Spring and continued to increase. Though no data are currently available, this may indicate that if nonnative fish populations are low and habitat manipulation is minimal, native fishes are able to dominate. In 1990, the population was estimated to contain approximately 12,000 individuals. In June 1994, however, Fish and Wildlife Service personnel visiting Hiko Spring observed relatively few Hiko White River Springfish and noted that algae were being removed from the spring pool (Donna Withers, Fish and Wildlife Service, pers. comm.). Even though Crystal Spring and its outflow stream were modified by the ditches of Native Americans prior to the arrival of the first European settlers in 1866, Hiko White River Springfish remained abundant into the I 960s (Courtenay et al., 1985). In 1959, largemouth bass were released into Crystal Spring by the Nevada Division of Wildlife (now Nevada Department of Wildlife, Courtenay et al., 1985). Largemouth bass moved out of the spring pool into the outflow channel, but did not reproduce and were not present in Crystal Spring or its outflow streams by 1961. Convict cichlids and shortfin mollies colonized Crystal Spring by invading from Ash Springs, and were very abundant in Crystal Spring by the 1970's (Courtenay & Deacon, 1982). The Hiko White River Springfish population declined sharply following the introduction of convict cichlids and shortfin mollies. As the nonnative species proliferated, Hiko White River Springfish numbers fell to a precariously low level due to predation and competition with the nonnative species. Hiko White River springfish continue to be very rare in Crystal Spring.
Recent information is taken from the 5 year review of the Springfish of the Pahranagat Valley (2012): "Currently, White River Springfish in the Ash Springs system occur primarily in the spring pools and outflow located above (to the east of) US 93, and in limited numbers in the outflow below US 93 to the confluence with Pahranagat Creek. The majority (~95%) of the fish’s distribution is on private property, with the remaining 5 percent being on land administered by the Bureau of Land Management (BLM). Population counts or estimates of White River Springfish have been inconsistent in methods and frequency throughout its monitoring history because of access issues related to land ownership; e.g., surveys have often been limited to the small portion of the fish’s distribution that is on BLM land. In 2005, NDOW was granted permission to access private lands and survey for White River Springfish throughout the Ash Springs system for the first time in about 10 years. Visual snorkel surveys conducted in 2006 found Springfish to be “abundant” and distributed throughout the BLM and private land portions of the spring and outflow. In 2010, NDOW counted 730 Springfish during a snorkel survey, and documented fish concentrating near the major spring inflows. In February 2011, 1,400 springfish were counted during an NDOW snorkel survey. During June 2012 snorkel surveys, NDOW counted 5,462 Springfish. Springfish were observed to be abundant throughout the spring outflow above US Highway 93 and rare in the outflow below the highway during all survey visits between September 2011 and June 2012. In Hiko Spring, the population increased and then remained fairly stable until the year 2000. During this period, the estimated population size reached a high of over 8,000 Springfish in 1986 and only occasionally fell below 4,000 fish. However, the Hiko Spring population has decreased substantially since 2000, coinciding with the appearance of red swamp crayfish (Procambarus clarkii) in the system. The estimated Springfish population at Hiko Spring has been between about 300 and 1,000 fish from 2006 onward. Population size estimates based on markrecapture methods at Crystal Spring in 1986 and 1987 were less than 300 individuals, and Springfish were restricted to the headwater pools (Tuttle et al., 1990). Surveys conducted by NDOW since 2004 indicate that the species continues to persist at this site, but at low numbers (typically, population estimates are <1,000 individuals in the north and south spring pools combined). While a variety of methods have been used to describe and estimate abundance of Hiko White River Springfish over the years, NDOW has recently conducted mark-recapture surveys using the Peterson Method with a 95% confidence interval recommended by Ricker (1975) to estimate population size. Population estimates for Hiko White River springfish at Hiko and Crystal springs during recent survey years are as follows:
2010 Population Estimates: Hiko Spring: 236 Springfish with a 95% confidence interval of 156 to 357 fish; Crystal Spring, North Pool: 228 springfish with a 95% confidence interval of 156 to 334 fish; Crystal Spring, South Pool: 490 Springfish with a 95% confidence interval of 252 to 681 fish.
2011 Population Estimates: Hiko Spring: 247 springfish with a 95% confidence interval of 147 to 448 fish; Crystal Spring, North Pool: 111 springfish with a 95% confidence interval of 69 to 191 fish; Crystal Spring, South Pool: 720 Springfish with a 95% confidence interval of 280 to 1,873 fish.
2012 Population Estimates: Hiko Spring: 379 springfish with a 95% confidence interval of 243 to 626 fish; Crystal Spring, North Pool: 63 springfish with a 95% confidence interval of 28 to 252 fish; Crystal Spring, South Pool: 407 springfish with a 95% confidence interval of 273 to 605 fish.
Due to the serious threats facing the Hiko White River Springfish populations, a refugium was created in 1984 by the Nevada Department of Wildlife, at Blue Link Spring in Mineral County, Nevada (Sevon & Delany, 1987). To protect the spring and its associated reservoir, 4.7ha of public land managed by the Bureau of Land Management were withdrawn from surface entry and mining. A total of 264 fish from Hiko Spring were released, quickly established a population, and remained abundant until 1990. A significant portion of the population was lost in 1990 when water flow into the reservoir decreased, either due to valve failure or vandalism, and the water cooled to a level lethal to Hiko White River Springfish. Following repair of the spring box water supply valves, the population was supplemented with an additional 150 fish from Hiko Spring. The fish recolonized the spring and the population has recovered to numbers comparable to pre-1990 levels, encompassing 5,500 specimens in 1994. A visual estimate during a July 2011 survey put the population at about 4,000 fish.
The Preston White River Springfish (Crenichthys baileyi albivallis) was found in the 1960s in six connected spring systems, but by 1991 it remained in only four. Cold and Lund Town springs are two systems from which Springfish have recently been extirpated. Cold Spring flows at 0.12m³/min, and Lund Town Spring, which flows at 0.9m³/min, is the southernmost and coolest (18.9°C; Maxey & Eakin, 1949) of the springs C. b. albivallis has been documented to inhabit. Except for Lund Town Spring, springs that support or have supported C. b. albivallis have water temperatures ranging from 21°C to 22°C. Reasons for the disappearance of this subspecies from these two springs are not definitively known, but both springs contain large populations of introduced guppies. Several nonnative fishes have been introduced into the White River system, but only Poecilia reticulata cooccurs with C. b. albivallis in Arnoldson and Nicholas springs (Scoppettone and Rissler, unpublished), and also inhabits Lund Town and Cold springs from which C. b. albivallis has just been extirpated. To get an idea of total numbers of Springfish from the Preston springs, sampling for a status report was conducted from 28 July to 12 August 1998 and from 5 to 12 January 1999. In the wider springhead region of Indian and Preston Big springs and the entire 10m length of Nicholas Spring, minnow traps approximately were spaced 4m apart in length and width. In the outflows of Indian, Preston Big, and Arnoldson springs, traps were set at 4m intervals along the length. In summer 1998, using mask and snorkel, fish in the 524m long outflow of Preston Big Spring were counted; in as much as the upstream most 64m was a broad spring pool, mark and recapture was employed. Lund Town Spring was sampled to further confirm C. b. albivallis extirpation from that system, but the surveyors were unable to contact the landowner to further confirm its extirpation from Cold Springs. It was estimated, that less than 5,000 Springfish exist in known habitats. Indian Spring had the greatest number, followed by Preston Big, Arnoldson, and then Nicholas springs. There was not a substantial difference in abundance between summer and winter. There was a greater number of fish during the summer in Arnoldson, but more in winter in Indian and Nicholas. A difference in numbers between the two seasons for Preston Big Spring could not be contrasted because of the different counting methods (snorkel and mark/ recapture) used.
The Moorman or Mormon White River springfish (Crenichthys baileyi thermophilus) is endemic to three small springs, the Moorman (or Mormon), Moon River and Hot Creek Spring. Williams and Wilde wrote about this subspecies in 1981: "Largemouth bass (Micropterus salmoides) entered Hot Creek Spring in the early 1970's from Dacey Reservoir, where they had been introduced as game fish. It was thought that the bass had extirpated the Springfish until personnel from the Nevada Department of Fish and Game discovered a group of Springfish separated from the main pool area by dense emergent vegetation. Hot Creek Spring was poisoned and the isolated Springfish were introduced into the main pool area after a barrier to prohibit entry of bass was repaired. Despite the barrier, one bass, probably introduced by fishermen, was observed in the main pool area in 1979. The proximity of bass to Hot Creek Spring can be expected to result in additional intentional or accidental introductions in the future. Viable populations currently exist in all springs inhabited by C. h. thermophilus. This subspecies formerly was common in the warm outflow of Hot Creek Spring. It has been extirpated there by largemouth bass." Here what Scoppettone et al. (2004) wrote about the result of a survey done at the beginning of the 1990's: "We observed tens of thousands of C. b. thermophilus on 10 June 1991 in the upper 100m of Hot Creek. The population had declined markedly to a count of only 5656 C. b. thermophilus by 25 July 1991 after an invasion of M. salmoides. By 12 September 1991 less than 50 C. b. thermophilus were observed hiding in emergent vegetation near the spring head. Crenichthys baileyi thermophilus was the only fish at Moon River and Moorman Springs."
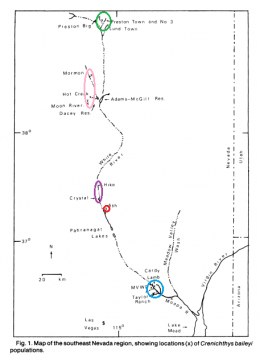
The left picture in the upper row shows the distribution of Crenichthys baileyi along the White River, marked the different suspecies sensu Williams and Wilde. The green ellipse circles the habitats of C. b. albivallis, the pink one those of C. b. thermophilus. In violet are marked the two springs where C. b. grandis is known from and the red circle marks the type locality of this species respectively from C. b. baileyi. With a blue circle are marked the habitats of C. cf. baileyi, described by Williams and Wilde as C. b. moapae. The right picture in the upper row shows a map of the springs of the Pahranagat Valley, while the left one in the lower row shows those in Preston historically known to inhabit Springfish. The right picture in the lower row shows a map of springs in the White River valley including those known for C. b. thermophilus.
In 1940 and 1941, Sumner and Sargent experimented with desert fishes out of different temperatured springs, among them Crenichthys baileyi from Preston spring (albivallis, T= 21°C) and Mormon spring (thermophilus, T= 37°C). They observed, that Preston spring fish "speedily died when transferred to Mormon spring. When the converse experiment is performed, they live in health for some days, and may perhaps do so indefinitely." They showed that fish from warm springs have a far higher rate of oxygen consumption than fishes of the same species living in a cool spring. The ratio, when fishes of approximately the same weight are compared, was not far from two to one.
In 2017, D. Cooper Campbell and Kyle R. Piller published a phylogenetic study of the Empetrichthyinae. Concerning Crenichthys baileyi, individuals from ten localities were included, namely the three springs where C. b. thermophilus occurs (Moon River Hot Spring, Moorman Hot Spring, Hot Creek), Crystal North and South Springs as well as Hiko Spring (representing C. b. grandis), Ash Sping (C.b.baileyi), Preston Big Spring (C. b. albivallis) and fish from the Muddy River and Hidden Valley Pond representing C. b. moapae. The studies were separatly done on the mitochondrial gene Cytochrome b and three nucleotide sequences, namely S7 intron-1, S8 intron-4, and P0 intron-1. Concerning cytb, the authors found only four clades. Three monophyletic clades representing the three subspecies C. b. baileyi, grandis and moapae (with moapae being a sister clade to a pair formed by grandis and baileyi) and a fourth clade composed of C. b. albivallis and thermophilus. Concerning the three introns, C. b. moapae was recovered as sister to all other C. baileyi populations. None of the subspecies within C. baileyi were separated by the TCS criterion into separate Haplotype networks. Phylogeny from species tree analysis based on cytb hypothesis following Bayesian statistics supports a model of two species. Taking in consideration genetic distances in the cytb gene of 1.3 to 1.7% that separate species in the Goodeinae and the result of the tree analyis, we follow a conservative approach here and recognize two species: Crenichthys baileyi from the type locality and the described subspecies C. b. albivallis, grandis and thermophilus, and Crenichthys cf. bailyei from the southern habitats in the Muddy River headwaters, C. b. moapae sensu Williams and Wilde.
Species of the subfamily Empetrichthyinae are oviparous fishes and share the lack of pelvic fins, while the subfamily Goodeinae is livebearing. Lynne Parenti (1981) finally proposed this subfamily as sister group to the Goodeinae and encompassed both in the family Goodeidae. Meanwhile the monophyly of the family Goodeidae and the narrow relationship between both subfamilies has been supported through several studies since the 1980's and is not doubted any longer.
Largemouth bass (Micropterus salmoides) entered Hot Creek Spring in the early 1970's from Dacey Reservoir, where they had been introduced as game fish. It was thought that the bass had extirpated the springfish until personnel from the Nevada Department of Fish and Game discovered a group of springfish separated from the main pool area by dense emergent vegetation. Hot Creek Spring was poisoned and the isolated springfish were introduced into the main pool area after a barrier to prohibit entry of bass was repaired. Despite the barrier, one bass, probably introduced by fishermen, was observed in the main pool area in 1979. The proximity of bass to Hot Creek Spring can be expected to result in additional intentional or accidental introductions in the future. Viable populations currently exist in all springs inhabited by C. h. thermophilus. This subspecies formerly was common in the warm outflow of Hot Creek Spring. It has been extirpated there by largemouth bass.
Husbandry:
Looking on the biotopes of Crenichthys baileyi, they suggest the species may prefer a habitat with moderate to swift current, structured with gravel, rocks, roots, branches, fallen leaves and river bank vegetation. Fry is rarely eaten, but it may depend on the quantity and quality of food and on the number of places to hide. When several different stages of juveniles occur, fry will be neglected, so it makes eventually sense to add separate brought up fry to the group with a size of 1.5 or 2cm to provide these stages and get a flock breeding colony.
The recommended tank size is at least 100 liters, bigger tanks with a generous base and little height (25cm are enough) are better for sure. With rocks and vegetation in the corners and backside of the tank well structured tanks combined with some roots and/or wood seem to do best with this species. The current should be moderate or swift, but the species can deal wih low oxygen values (down to 3mg/l).
In the wild, the species feeds mainly from small or middle-sized invertebrates like bloodworms or insect larvae, gammarids, ostracods, but also algae, aufwuchs, detritus and other organic matters, so feeding with similar food, water fleas, Mysids and other food from animalistic sources as well as stones and pebbles covered with green algae will be best for this omnivorous fish. In aquarium, it feeds very well from flake food, granulate and tablets, additionally given Nauplia of Brine Shrimps are eaten greedy. Additionally, as aufwuchs and green algae are taken in the natural habitat, providing some kind of vegetables like boiled peas is a good food supplement. The species is not shy.
Concerning water quality, this species is in need of bigger water changes (60-80% every week) like most of the Goodeids, especially river and spring inhabiting species, so an automatic water changing system can be helpful, but in contrast to most of the Goodeid species from Mexico, it needs to be temperated and the oxygen level could be low. This species does best at temperatures between 20 and 29°C (depending on origin of the population) though it is able to withstand lower temperatures down to even 12°C for a short period.
This species is doing very well when is kept in the open from spring to fall, starting when the water temperature by night does no more deceed 18°C and colder periods are no longer expected. Bring them out in the early afternoon, the time of the day with the highest water temperature. Bring the fish in before the water temperature deceeds 18°C by night and keep them at this temperature for the first days, then slowly raise the temperature to the convenient range of this species over the winter time.
Populations in holding:
Here each species are assigned populations of fish in husbandry and in brackets aliases of these locations to assist in identifying own stocks. Each population is assigned a unique Population-ID, composed by the ESU, the subbasin where this population is occurring (three capital letters) and a unique location identifier.
Populations in holding:
1. Creba1-WHR-ASpr
Population: Ash Springs (aka Crenichthys baileyi baileyi)
Region: Lower Colorado Basin
Accounting Unit: Lower Colorado-Lake Mead
Cataloging Unit: White River
Locality: sping area at Ash Springs, Pahranagat Wash
2. Creba2-WHR-HiSpr
Population: Hiko Spring (aka Crenichthys baileyi grandis)
Region: Lower Colorado Basin
Accounting Unit: Lower Colorado-Lake Mead
Cataloging Unit: White River
Locality: Hiko Spring, Pahranagat Wash